Suche
Lesesoftware
Info / Kontakt
Electrowetting - Fundamental Principles and Practical Applications
von: Frieder Mugele, Jason Heikenfeld
Wiley-VCH, 2018
ISBN: 9783527412419 , 350 Seiten
Format: ePUB
Kopierschutz: DRM




Preis: 97,99 EUR
eBook anfordern 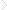
1
Introduction to Capillarity and Wetting Phenomena
The goal of electrowetting () is to manipulate small amounts of liquid on solid surfaces by tuning the wettability using electric fields. Many phenomena encountered in EW experiments are actually not special to electro wetting and electro capillarity. They are simply wetting and capillary phenomena that can be observed in many circumstances with surfaces of more or less complex geometry and more or less complex distributions of wettability. EW is rather unique in its ability to change contact angles over a very wide range in a very fast and usually very reproducible manner. Therefore, EW has enabled an unprecedented degree of control of drop shapes and dynamics – and along with it a plethora of possible applications. Nevertheless, many basic observations in EW are still variants of classical wetting and capillary phenomena and hence subject to the general laws of capillarity. To understand the phenomena that we encounter in EW experiments and to be able to fully exploit the potential of EW technology, we therefore need a good grasp of the physical principles of wetting and capillarity. The purpose of this first chapter is to introduce the reader to these basic concepts. We will discuss in Section 1.1 the microscopic origin of surface tension and interfacial energies starting from molecular interaction forces. In Sections 1.2 and 1.3, we introduce the two basic laws governing the mechanical equilibrium of liquid microstructures, the Young–Laplace law and the Young–Dupré equation. In Section 1.4, gravity is added as an additional external body force. Section 1.5 is devoted to a concise but somewhat formal mathematical derivation of the fundamental equations that were discussed in Sections 1.2–1.4. Less mathematically interested readers can skip that section without missing important physical results. In Section 1.6, we address aspects of wetting on the nanoscale and introduce concepts such as the disjoining pressure and the effective interface potential that are relevant, among others, in the vicinity of the three‐phase contact line and for the stability of nanometric films. In Section 1.7, we discuss some consequences of surface heterogeneity such as contact angle hysteresis and superhydrophobicity. In order to provide the reader with an intuitive understanding of the theoretical concepts and formulas, we will discuss a variety of classical capillary phenomena frequently switching between force balance arguments and considerations based on energy minimization.
A deeper discussion of many aspects addressed here can be found in the excellent textbook by deGennes et al. [1] that we will refer to multiple times.
1.1 Surface Tension and Surface Free Energy
Capillary and wetting phenomena are important on small scales. Small scales always imply large surface‐to‐volume ratios: the smaller the system, the larger the fraction of atoms or molecules that is located at interfaces. This is the fundamental reason why interfacial effects are crucial in all branches of micro‐ and nanotechnology, including micro‐ and nanofluidics. Being at the surface means being within the range of molecular interaction forces of the geometric interface. Using a typical value of, say, Δr=1 nm we can simply estimate the fraction of molecules in a small drop that is affected by the interface: The volume of a drop or radius r is V=4πr3/3. The volume of a shell of thickness Δr is Vs=4πr2Δr. Hence the ratio is ΔVs/V=Δr/3r. For a millimeter‐sized drop, one molecule in three million is thus at the surface. For a micrometer‐sized drop, the ratio is one in three thousand. These surface molecules, not the ones inside the bulk of the drop, determine the equilibrium shape of the drop.
1.1.1 The Microscopic Origin of Surface Energies
Throughout this book, we will consider liquids as continuous media characterized by material properties such as density, viscosity, and cohesive energy. Interfaces between two different phases such as liquid and vapor or liquid and solid are characterized by an interfacial energy or tension γ. For the specific interface between a liquid and its own vapor, it is common to speak of surface energy or surface tension. Both expressions, interfacial energies and tensions, mean the same thing, and we will use them interchangeably throughout this book. A surface energy is an excess free energy per unit area of the surface. It is measured in Joule per square meter or more frequently in milli Joule per square meter because the latter turns out to be more convenient for most common liquids. A surface tension is a tensile force per unit length acting along the surface. It is measured in Newton per meter, or in milli Newton per meter, which is dimensionally equivalent an energy per unit area. (In older books, you will sometimes find surface tensions reported in dyn/cm, which is numerically equivalent to mN/m.) These two complementary perspectives have their origin in complementary experimental observations and conceptual approaches. As often in mechanics, a given problem can be considered either from the perspective of energy minimization or from the perspective of force balance. Both views are perfectly equivalent. As classical theoretical mechanics tells us, Newton's equations of motion are the differential equations that any solution of a mechanical problem has to fulfill in order to minimize the Lagrangian and thus, in equilibrium, the energy of the system. Whether energy minimization or force balance is more convenient or more intuitive to solve a specific problem depends on the problem at hand – and to some extent on personal taste.
Why is there an excess energy associated with an interface? To understand this point, it is convenient to deviate for once from our general continuum picture and to instead consider the individual molecules and their mutual interaction. Let us first look at a reference molecule somewhere in the bulk of the liquid drawn in black in the bottom of Figure 1.1a. The reference molecule interacts with its neighbors via some molecular interaction potential. The details of this potential are characteristic for each specific liquid. While all relevant molecular interactions are fundamentally electromagnetic in nature, there are different flavors such as direct Coulomb interactions between charged ions, charge–dipole interactions, dipole–dipole interaction, van der Waals interaction, etc. A detailed discussion can be found in classical textbooks of surface forces, such as the ones by Butt and Kappl [2] and by Israelachvili [3]. Notwithstanding all the details, the generic shape of the interaction potentials usually looks as sketched in Figure 1.1c. There is a strong repulsive barrier at short distances that prevents the molecules from overlapping, and there is an attractive force with a range of typically a few molecular diameters that depends on the specific interaction. The minimum of the potential determines the average separation of the molecules and thus the density of the fluid. In practice liquids are very dense, and molecules continuously bounce into the repulsive potential barrier. The target molecule interacts with all its neighbors (drawn in gray) within the range of the interaction potential, as indicated by the dashed circle in Figure 1.1a. The sum of the interaction energy with all the neighbors within this range determines the cohesive energy Ecoh of the liquid. This is the reference energy of a molecule in the bulk. If we now consider a second molecule close to the surface, it is obvious that that molecule lacks binding partners above the liquid surface. As a consequence, it has less binding energy than the reference molecule in the bulk. This lack of binding energy constitutes an excess energy as compared with the reference state. If we denote the area per molecule at the interface as a2, we obtain an estimate for the surface energy based on a very generic atomistic picture:
Figure 1.1 (a) Schematic illustration of one specific molecule (black) in the bulk liquid interacting with neighbors (gray) within the range of interactions of the molecular forces (dotted circle) and a second molecule close to the interface that is missing binding partners on the opposite side. (b) Density profile at the liquid surface with a gradual transition from the bulk liquid density to the vapor density. (c) Typical molecular interaction potential between two molecules with a minimum close to the molecular diameter a.
We thus expect the surface tension to scale with cohesive energy of the liquid. Table 1.1 gives an overview of the surfaces tensions as well as other properties of liquids commonly used in EW. Generally, more polar molecules (stronger dipole, stronger Coulomb forces) exhibit stronger attractive forces. Hence, they typically display a higher cohesive energy, higher boiling point, and higher surface tension. Water with its very large dipole moment is an example of a liquid with a particularly high cohesive energy in view of its low molecular weight. Even stronger cohesive forces are found for liquid metals, for which the...





